April SonoProps
Hello everyone, welcome to the April 2025 SonoProps!
This month’s first SonoProps goes to Dr. Eytan Mendelow.
Dr. Mendelow performed on a scan on one of Dr. Badoolah’s patients, a late 50’s male that presented to the ED with acute onset of painless vision loss in his right eye. A stroke alert was called and the CT’s were negative. A Maestro exam was performed that did not demonstrate a “cherry-red” spot but did demonstrate significant vascular attenuation.
The POCUS clips below demonstrate Dr. Mendelow’s findings. The patient was admitted to the stroke service.
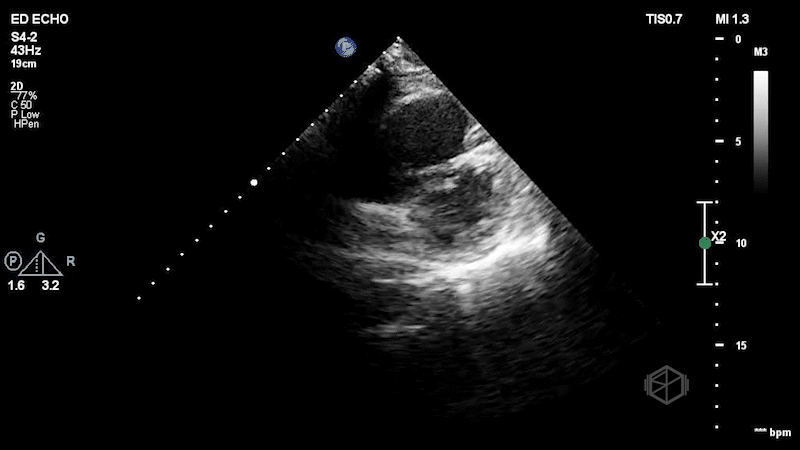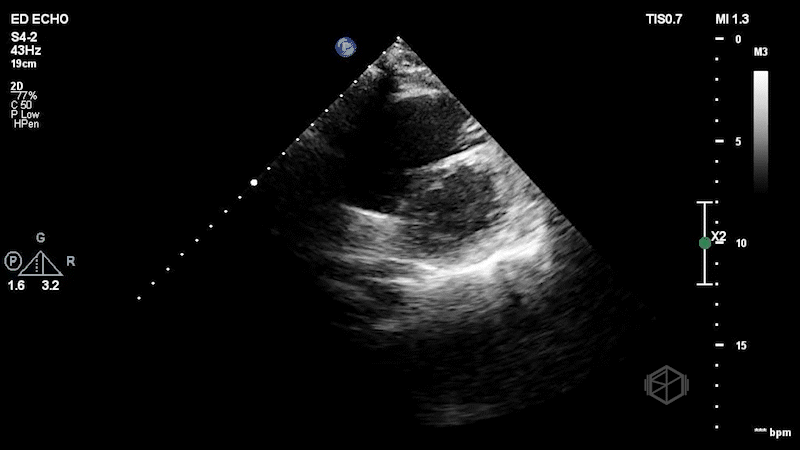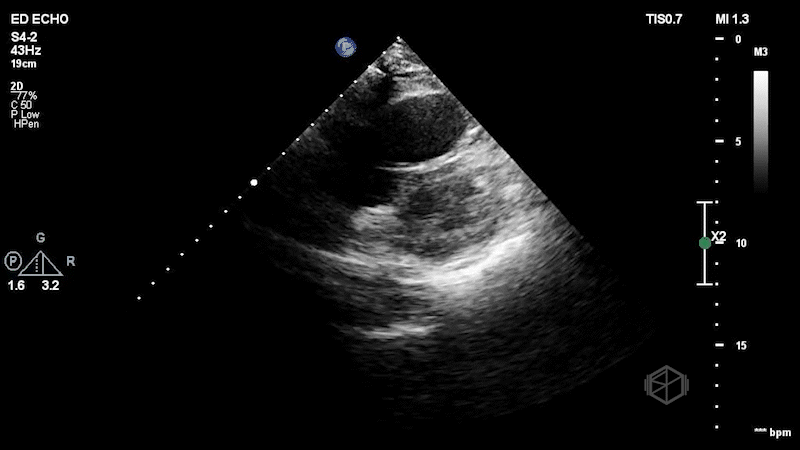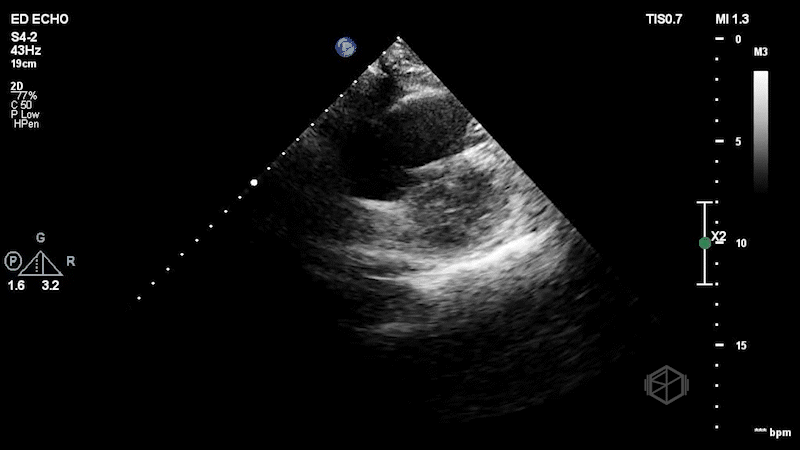
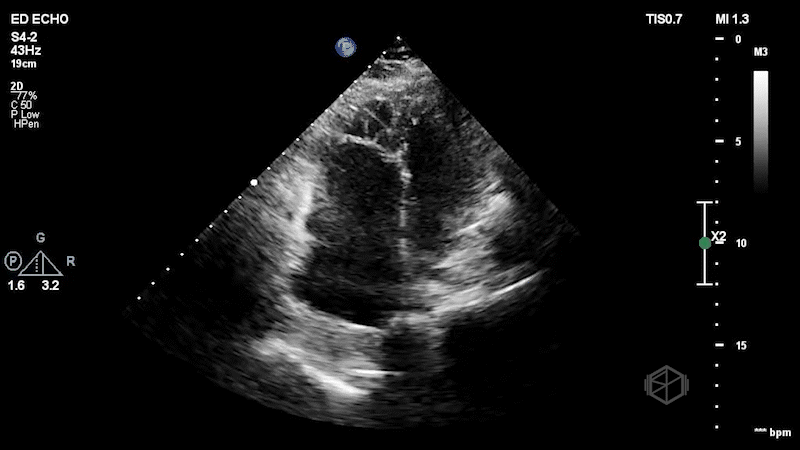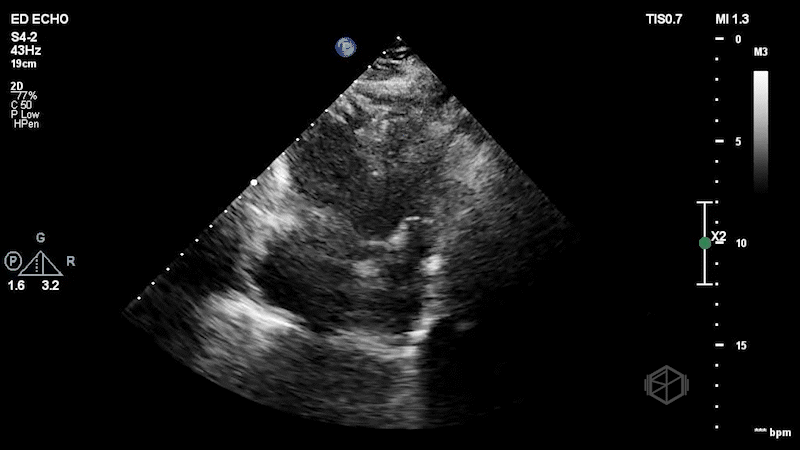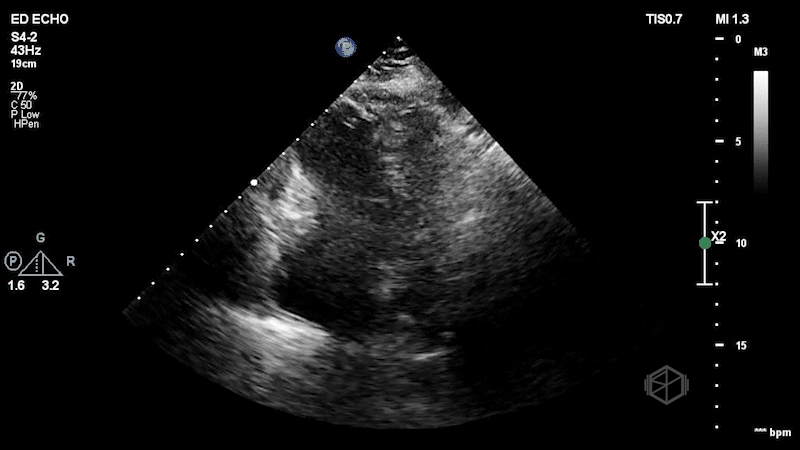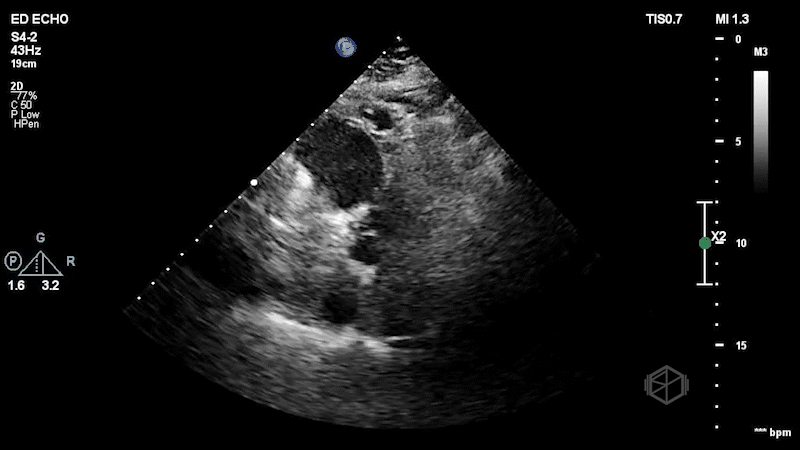
The first clip demonstrates a thin hyperechoic flap that does not attach to the optic nerve consistent with a posterior vitreous detachment. The second clip highlights why it is important not to anchor on only one diagnosis. The second clip demonstrates a hyperechoic dot/spot near the optic nerve. A spectral doppler exam could have been performed although keeping in mind ALARA, it should not be performed for too long to avoid damaging the sensitive cones and rods of the eye.
Diagnosis: Central retinal artery occlusion, posterior vitreous detachment
Learning points:
A posterior vitreous detachment (PVD) is often faint on normal gain, but becomes more noticeable with higher gain. It is a thin wispy flap that is not attached to the optic nerve that moves with oculo-kinetics. Sensitivity for diagnosing PVD is lower than red, likely due to clinicians being more comfortable diagnosing retinal detachment (30977855, 29042095). If the flap is close to the posterior aspect of the eye, PVD vs. RD may be more difficult to distinguish.
Despite seeing the obvious PVD, it is good not to anchor on just one diagnosis. PVD should not cause vision loss, so other diagnoses should be considered.
CRAO can be diagnosed by evaluating for the presence of a hyperechoic "spot sign" near the optic nerve, within the central retinal artery (30775656, 36895673, 34367764).
In one small prospective study, the hyperechoic "spot sign" was visible in 10 of 12 patients (83 %) with embolic CRAO. The detection of embolic CRAO using the "spot sign" had a sensitivity of 83 % and a specificity of 100 % (23023446).
In a larger study, a spot sign was seen in 32 of 46 of patients with CRAO and in 7 of 23 patients with branch retinal artery occlusion (BRAO). Interobserver agreement was excellent (Cohen`s kappa 0.98). Giant cell arteritis can also cause CRAO, but the hyperechoic spot sign is not visible in those patients (33577572).
Spectral doppler can also be used to not the absence of arterial flow near the hyperechoic spot sign, although this should be used with caution as noted above.
The second SonoProps goes to Dr. Hardeep Singh. Dr. Singh is rotating at Suffolk, demonstrating how POCUS makes a difference in early diagnoses. Dr. Singh evaluated a mid-60-year-old female who presented to the ED with shortness of breath and hypoxia. With multiple differentials to consider, he quickly performed a POCUS that made his diagnosis. The echo clips are shown below:
The patient has a dilated right ventricle obvious even in the parasternal long. The parasternal short view demonstrates a dilated right ventricle with a D-sign that is mostly persistent indicating RV pressure overload. The apical 4 view demonstrates apical hyperkinesis with lateral wall hypokinesis (McConnell’s sign), and the IVC is plethoric with minimal respiratory variation. Dr. Singh previously got a SonoProps for this diagnosis.
The expedited CTPE was read as “large filling defect is present in the distal left main pulmonary artery with extension into the left upper lobe branches as well as arterial branches of the left lower lobe. Filling defect is also seen within the right lower lobe pulmonary artery just distal to the bifurcation. Filling defects are present within multiple segmental branches of the right lower lobe and the right middle lobe. Filling defects are also visualized within the right upper lobe branches. There is no evidence of right ventricular strain.” Clearly, there was evidence of right ventricular strain on echo. The patient was taken for a thrombectomy.
Diagnosis: Massive pulmonary embolism with right heart strain
Learning point:
In patients with suspected or confirmed pulmonary embolism, emergency physician-performed bedside echocardiography showed high specificity (98%) and a strong positive predictive value (88%) for right ventricular dilatation, though with limited sensitivity (50%), supporting its use in rapid decision-making for high-risk patients in the ED (24075286).
In another study, ICU fellows accurately identified right ventricular dysfunction in acute pulmonary embolism with an area under the curve of 0.83, outperforming biomarkers like troponin (AUC 0.70) and BNP (AUC 0.62), while reducing time to assessment significantly compared to formal transthoracic echocardiography (28953498).
ED POCUS identified right ventricular strain (RVS) as a significant independent predictor of in-hospital adverse outcomes, with RVS increasing the odds of complications by 9.2 times (OR 9.2, 95% CI 3.2–29) and occurring in 64% of those with adverse events compared to 16% without (23827166).
CTPE is pretty good at identifying right heart strain with high sensitivity but moderate specificity for RVS compared to TTE. RVS on CT and TTE predicts more events than either alone. TTE provides extra prognostic value over CT for predicting post-PE clinical deterioration (27664798, 34245115).
Keep in mind — there are also other findings that may cause right heart strain on echo, such as cor pulmonale from COPD or chronic right heart failure etc. As radiology always states, “correlate clinically” and compare with prior echoes if available.