October SonoProps
Welcome to another month’s SonoProps. This month had many great scans by our ultrasound team and our ED team, but there can only be two.
So our first SonoProps goes to our ultrasound team this month, Dr’s Gena Koutsounadis and Obioma Nkemakolam.
They were examining a 30’s female who was presenting to the ED with headaches for multiple years and had some vision disturbances. The performed an ocular POCUS that demonstrated the following:
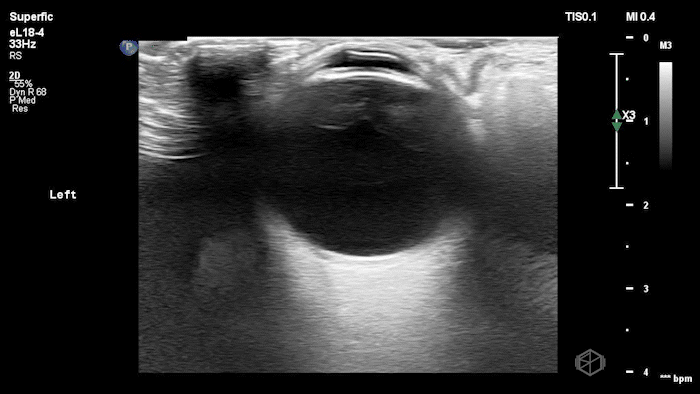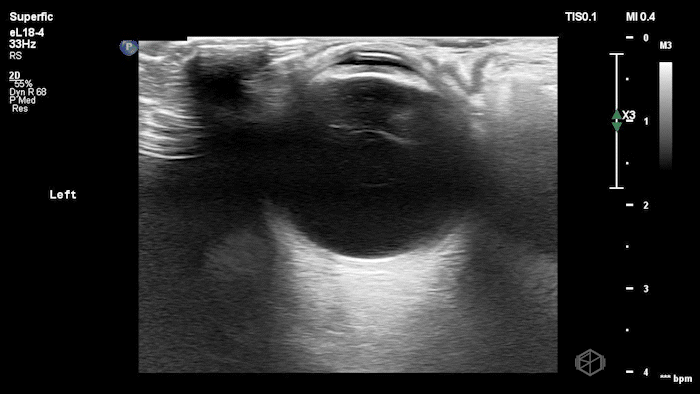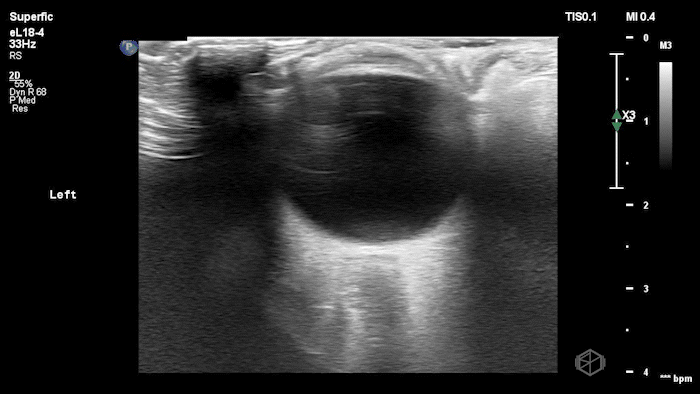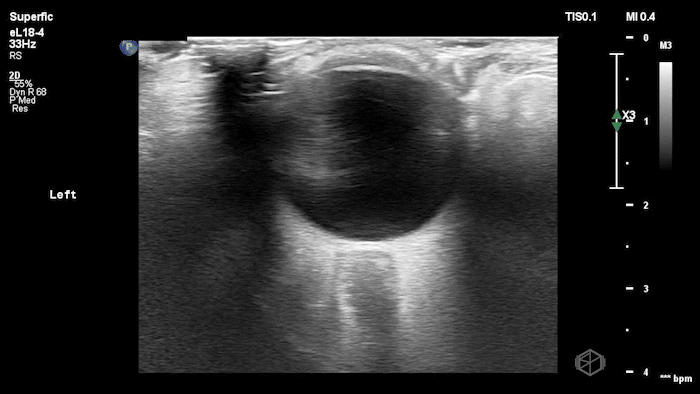
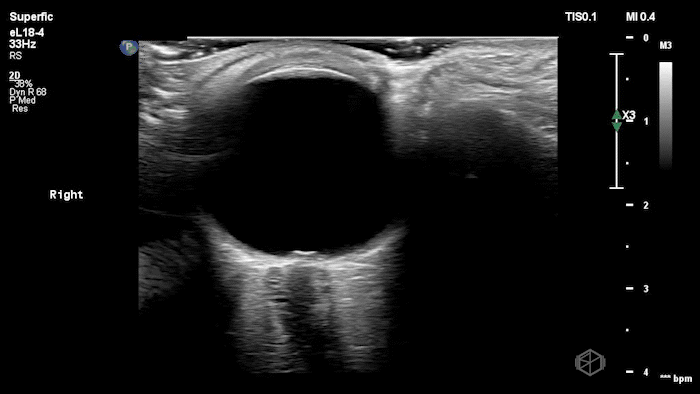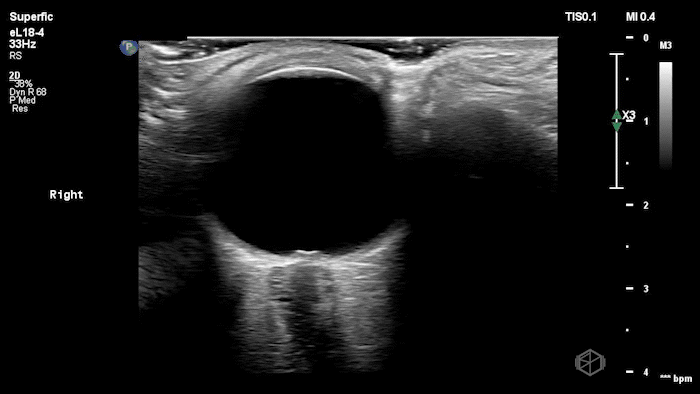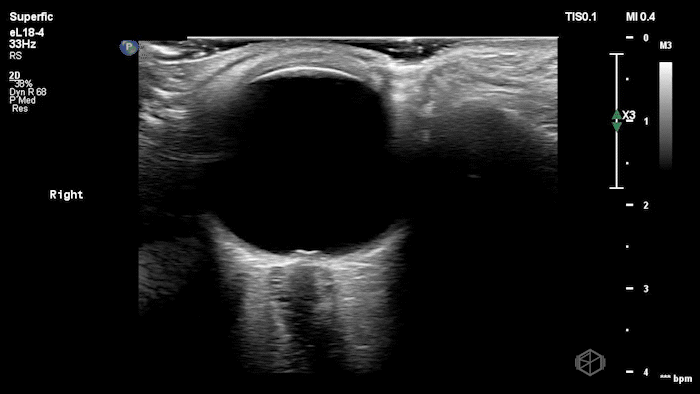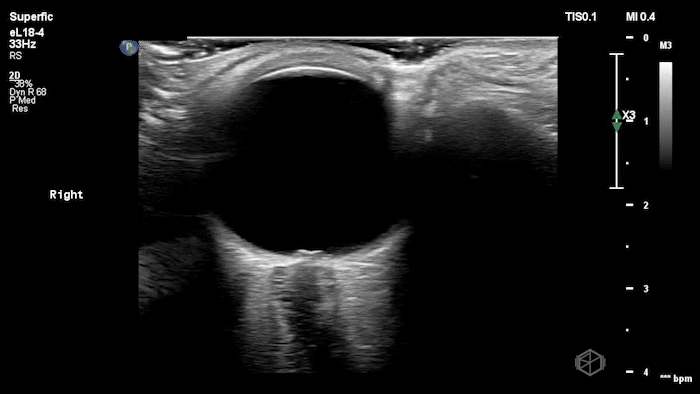
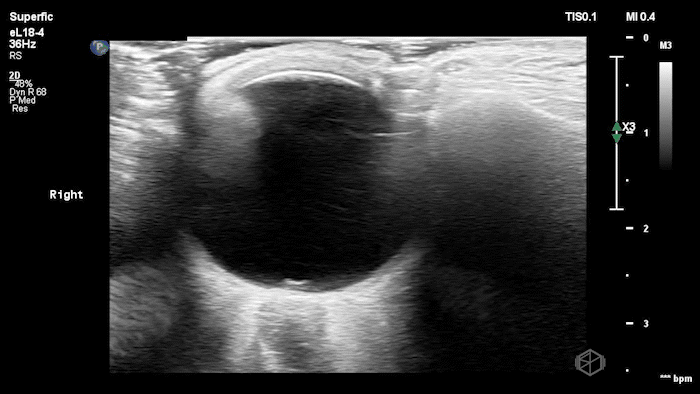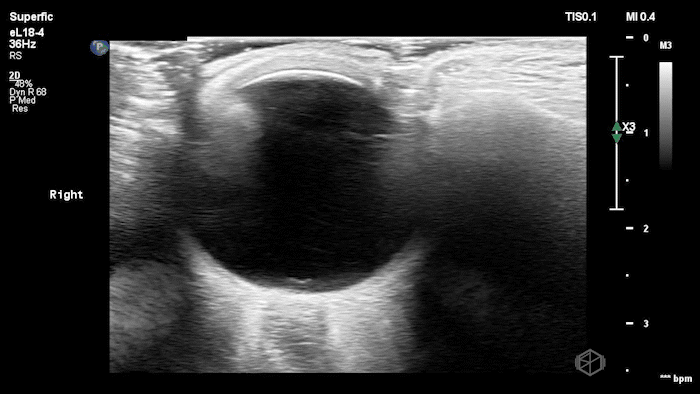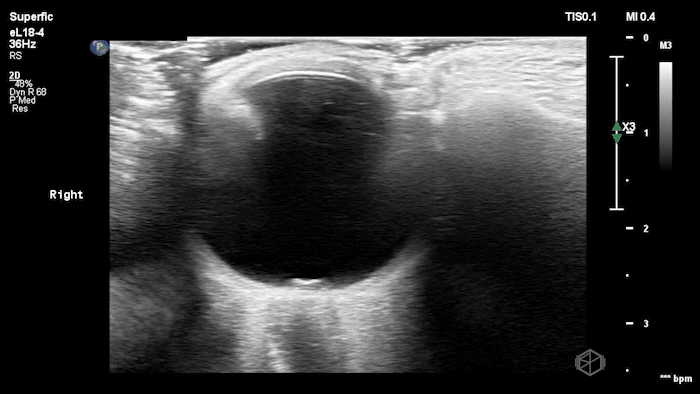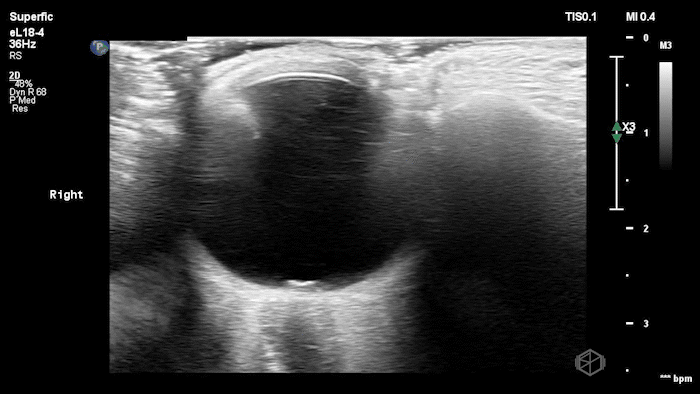

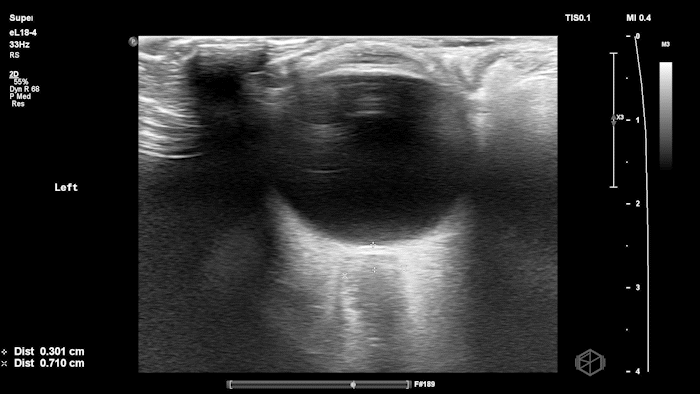
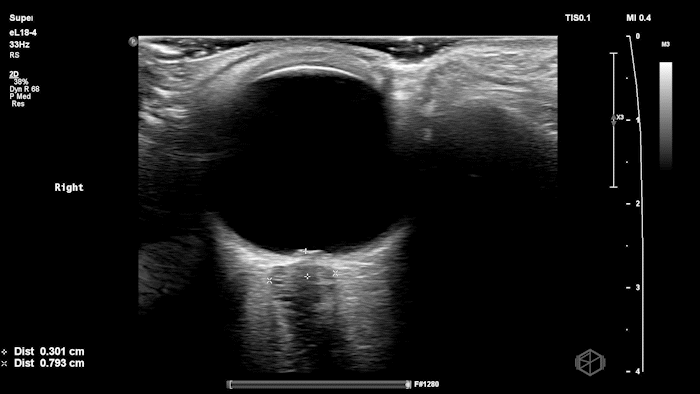
The POCUS shows optic nerve edema right (0.793cm) greater than left (0.71cm) with bilateral papilledema. There may also be a faint vitreous detachment of the right eye. There was concerned for increased intracranial pressure.
The patient had an MRI brain and orbits that showed, “intraocular protrusion of the right greater than left optic nerve heads with increased prominence of the CSF spaces of the bilateral optic nerve sheaths, can be seen in a idiopathic intracranial hypertension.” She had a lumbar puncture that had elevated opening pressure and was started on acetazolamide.
Diagnosis: Bilateral optic nerve edema/papilledema and idiopathic intracranial hypertension
Learning points:
ONSD measurements in this case are markedly abnormal. The optic nerve sheath diameter (ONSD) is measured 3 mm posterior to the globe. In adults, an ONSD > 5.0 mm is generally considered abnormal; values > 6.0 mm are strongly associated with raised intracranial pressure (ICP). Bilateral measurements of 7.9 mm (right) and 7.1 mm (left) are consistent with elevated ICP. The probe should be held lightly with ample gel to avoid artifactual compression (📚 PMID: 18509619, 18275454)
Ocular ultrasound demonstrates strong diagnostic performance for raised ICP. Meta-analyses show high accuracy: pooled estimates around ~90% sensitivity and ~80–90% specificity, with performance varying by cutoff and population; ONSD should be used as an adjunct to clinical assessment and neuroimaging. (📚 PMID: 21505900, 30019201)
Papilledema may lag behind acute ICP elevation. ONSD can enlarge rapidly with ICP rise, whereas fundoscopic papilledema often develops later (typically ≥24 hours and usually within ~7 days). Absence of papilledema does not exclude early intracranial hypertension. (📚 PMID: 35698673)
Point-of-care US measurement of optic disc elevation/height helps when fundoscopy is limited. Studies report cutoffs from ~0.6–1.0 mm: ≥1.0 mm is highly specific for papilledema in ED cohorts; lower cutoffs (≈0.6–0.7 mm) improve sensitivity. (📚 PMID: 24050798, 37227512)
The next SonoProps goes to Dr. Andrew Weinberger. He performed a scan on a mid-70’s female with a past medical history of hypertension, diabetes, hyperlipidemia that had neck pain and then started having chest pain.
His scan showed the following:
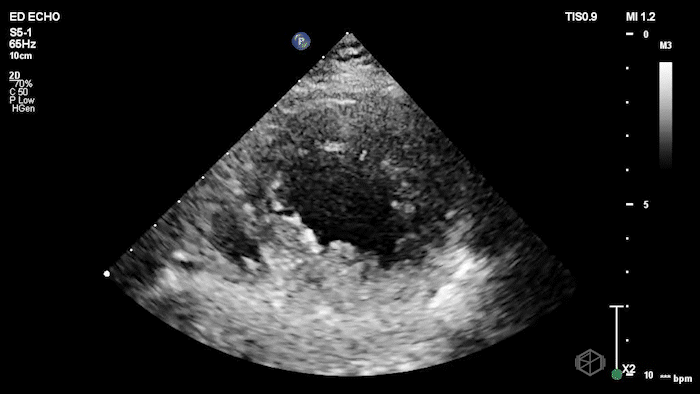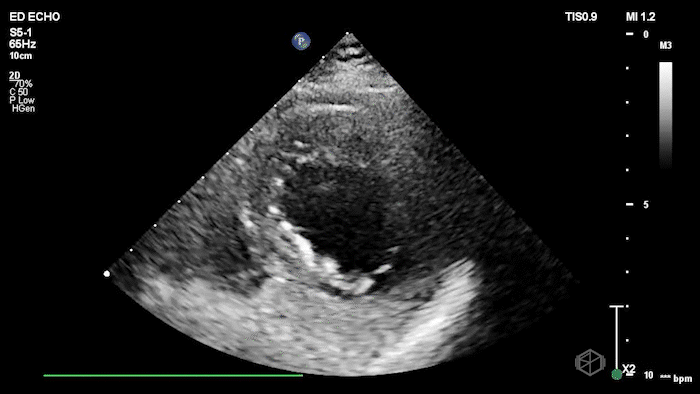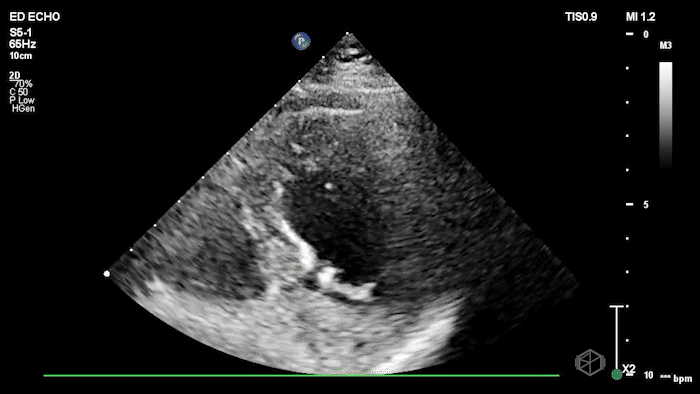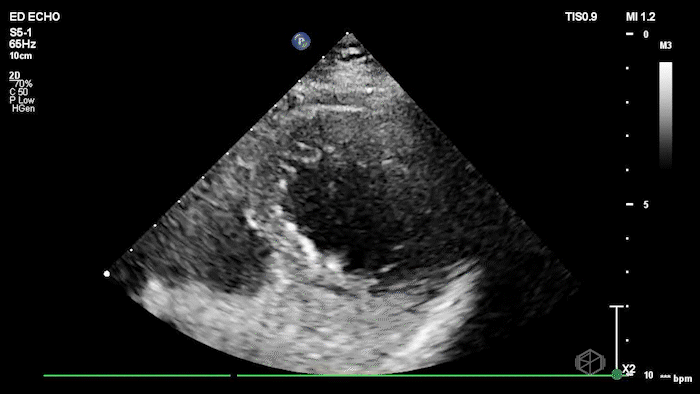
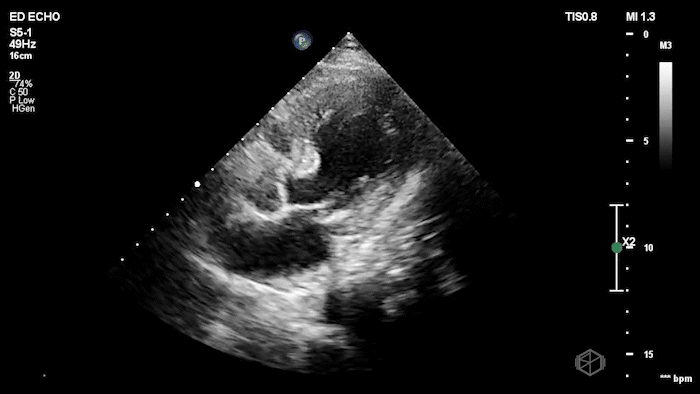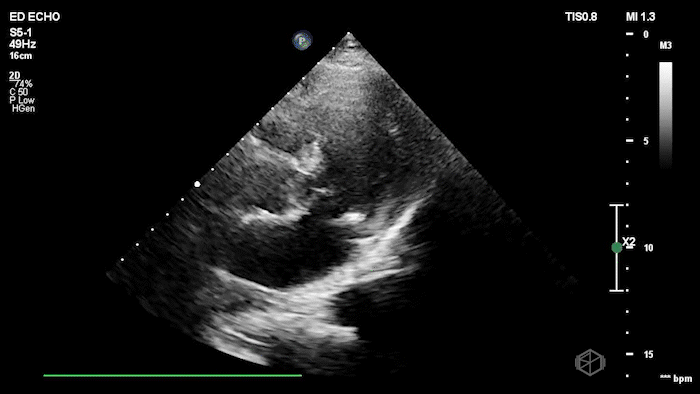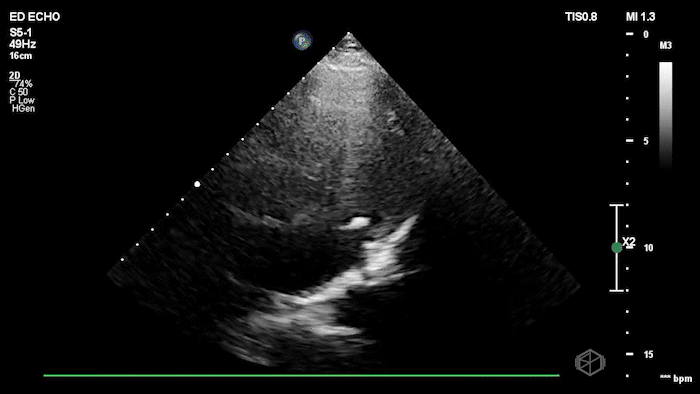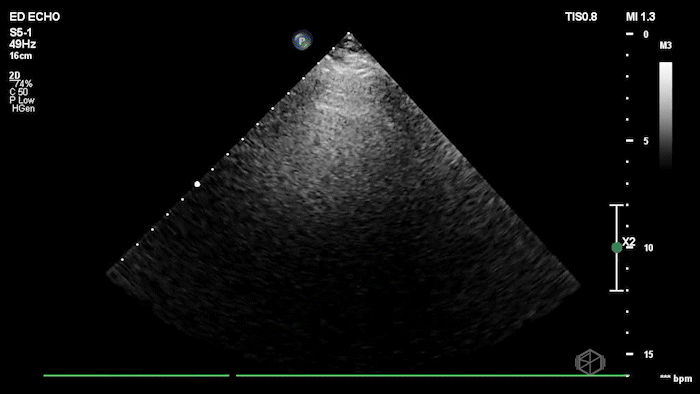
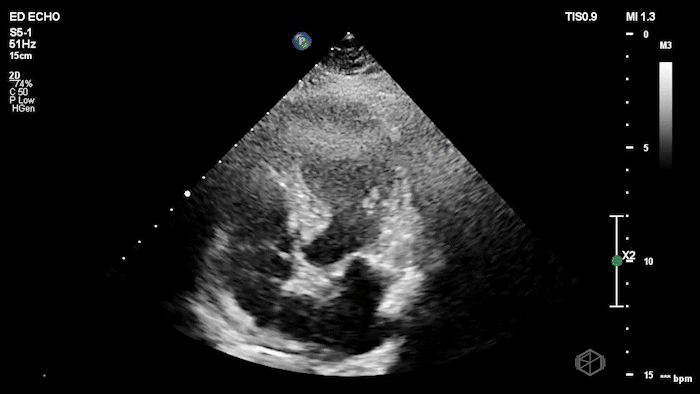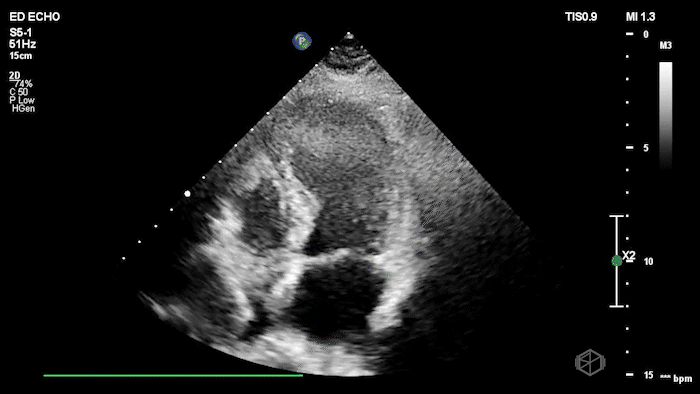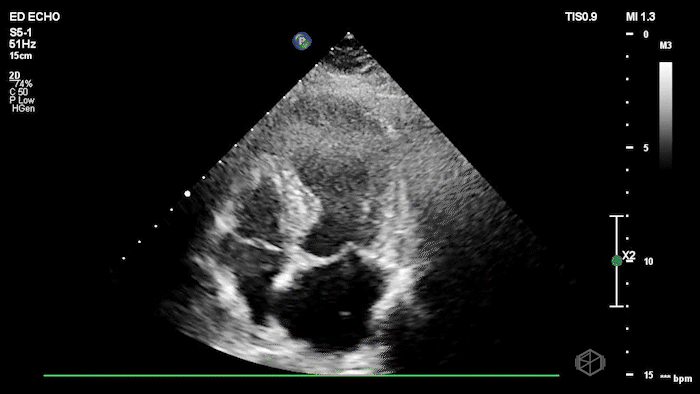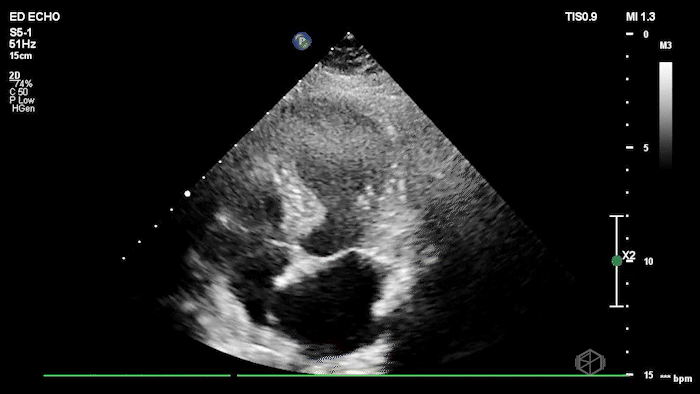
The echo shows a reduced ejection fraction with an antero-septal wall motion abnormality.
The patient’s EKG demonstrated an antero-septal AMI.
The patient was taken for a cath and found to have a 100% LAD occlusion. The ejection fraction was 20-29%.
Diagnosis: AMI - Anteroseptal LAD 100%
Learning points:
RWMA shows up early and maps the culprit. In acute ischemia, new regional wall-motion abnormality (RWMA) appears before—or despite nondiagnostic—ECG changes. In STEMI cohorts, EM residents with core ultrasound training detected RWMAs with sens 88% / spec 92% vs comprehensive echo, and territory agreement was substantial—useful for rapidly localizing an LAD-territory infarct like this case. (📚 PMID: 30987914)
High specificity helps you “rule-in” in non-ST presentations, but don’t “rule-out” with a normal scan. In ED chest-pain patients without ST-elevation, POCUS-detected RWMA predicted NSTE-ACS with spec ~93% but sens ~43%—great when present, insufficient to exclude ACS when absent. Treat a normal study as low likelihood, not a discharge ticket (📚 PMID: 38985842).
Residents and EM attendings can learn this and perform well. A 30-minute teaching module improved EP accuracy for WMA recognition from 67% → 87% on test images (pre/post). In clinical ACS cases, residents achieved sens ~86% and attendings/fellows ~85% for RWMA vs cardiology echo (specificity higher in more experienced operators). (📚 PMID: 22046540)
When new RWMA is seen by EPs in patients who don’t meet initial ECG cath-activation criteria, it closely matches cardiology echo findings (EP RWMA vs cardiology echo: spec 96%) and is highly specific for patients who will undergo intervention/CABG (spec ~93%, sensitivity modest). Use ‘new RWMA’—relative to a prior normal echo—as a trigger for expedited cardiology involvement. (📚 PMID: 38205979)
Common pitfalls: Old infarcts/cardiomyopathy can mimic “new” RWMA; paced rhythms/LBBB can confound ECG, making echo relatively more helpful. Suboptimal windows and tachycardia can hide subtle hypokinesis—optimize gain, depth, and frame-by-frame endocardial tracking; when in doubt, label as “possible RWMA—needs cardiology echo.”